Kaiser Permanente Vaccine Study Center found benefit over standard-dose inactivated influenza vaccine
A higher-dose flu vaccine using recombinant technology was associated with 15% fewer flu cases in older patients than the commonly used, standard-dose flu vaccine. The study, carried out by the Kaiser Permanente Vaccine Study Center, compared Flublok with standard-dose flu vaccine that is made using chicken eggs.

The study was published in the New England Journal of Medicine on December 13.
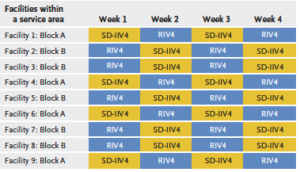
The researchers randomized a total of 2.4 million Kaiser Permanente Northern California (KPNC) patients ages 18-64 to receive either the higher-dose recombinant vaccine or the standard-dose egg-based vaccine during the 2018 to 2021 influenza seasons. However, data from the third season — 2020-2021 — was ultimately excluded from the analysis because there was virtually no flu circulating that year, in part because of the COVID-19 pandemic. That left 1.6 million patients in the analysis.
Among the older patients ages 50 to 64, those who received the recombinant vaccine were 15.3% less likely to have a lab-confirmed case of flu than those who received the standard-dose vaccine (known as quadrivalent standard-dose inactivated influenza vaccine). There was not a significant difference in which patients had a flu-related hospitalization, likely because there were relatively few hospitalized patients to analyze.
“This large study adds to what we know about relative effectiveness of flu vaccines, which are given to millions of Americans each year, and on which we rely to reduce illness and hospitalization, particularly among vulnerable groups,” said lead author Amber Hsiao, PhD, MPH, project manager with the Kaiser Permanente Division of Research.
Flublok has a higher dose of flu antigen than the standard-dose, quadrivalent vaccine. The results from the 2 flu seasons analyzed suggest Flublok’s better protection is more likely related to its higher dose of flu antigen than to manufacturing differences, Hsiao said.

The study compared the relative effectiveness of the 2 vaccines against one another, but did not estimate how effective each was against flu when compared with people who were not vaccinated. In the older group ages 50 to 64, the analysis found 2 patients per 1,000 tested positive for flu after receiving Flublok, while 2.34 patients per 1,000 tested positive after receiving the standard-dose quadrivalent vaccine.
Strengths of the study included the large number of patients and the novel randomized design, said senior author Nicola Klein, MD, PhD, director of the Kaiser Permanente Vaccine Study Center. All KPNC facilities provided one of 2 flu vaccines alternately each week of the flu season. Which vaccine patients received depended upon which facility and week they sought vaccination. The researchers then looked for patients who tested positive for flu and for flu-related hospitalizations.
Varying effectiveness
Influenza causes mild to severe illness in 9 million to 41 million people each year in the U.S. Vaccines vary in their effectiveness year to year, depending on whether public health officials have chosen strains of influenza virus that match what eventually circulates in the population during flu season. Effectiveness can vary from 20% to 60%, Klein said. “It is clear that research into more consistently effective flu vaccines is needed,” she said. “This is one of the main areas of work for our Vaccine Study Center.”
Flu seasons have been unusual the past few years because of the COVID-19 pandemic; restrictions on public interactions greatly reduced the transmission of flu virus. This study examined the 2018/2019 and 2019/2020 winter flu seasons in the U.S., which included 1.6 million of the total 2.4 million vaccinated at KPNC, when case numbers were more typical.
The study did not include patients 65 and older because most were receiving a third type of vaccine called Fluzone High-Dose, which is also a high-dose flu vaccine and uses the chicken egg method. Last flu season (starting in August 2022), the Advisory Committee on Immunization Practices recommended that older adults receive a high-dose (Fluzone High-Dose or Flublok) or adjuvanted flu vaccine (Fluad).
The study was funded by Sanofi.
Other co-authors were Arnold Yee, MBA, Bruce Fireman, MA, John Hansen, MPH, and Ned Lewis, MPH, all of the Vaccine Study Center.
# # #
About the Kaiser Permanente Division of Research
The Kaiser Permanente Division of Research conducts, publishes and disseminates epidemiologic and health services research to improve the health and medical care of Kaiser Permanente members and society at large. It seeks to understand the determinants of illness and well-being, and to improve the quality and cost-effectiveness of health care. Currently, DOR’s 600-plus staff is working on more than 450 epidemiological and health services research projects. For more information, visit divisionofresearch.kaiserpermanente.org or follow us @KPDOR.




Comments (0)