Vaccine Study Center to lead effort searching medical records for serious reactions
[Story updated on March 1, 2020]
New vaccines recently approved to protect against COVID-19 disease were developed at record speed. To support public confidence in the massive vaccination effort under way throughout the country, it will be particularly important to track any potential serious reactions in patients who are inoculated. Kaiser Permanente’s Vaccine Study Center is leading a key part of the national safety surveillance effort.
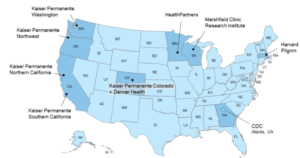
The study center, part of the Kaiser Permanente Northern California Division of Research, was chosen by the Centers for Disease Control and Prevention (CDC) to carry out a Rapid Cycle Analysis (RCA) through CDC’s Vaccine Safety Datalink (VSD) network. Organizations that share data through the network include 5 Kaiser Permanente regions, HealthPartners in Minneapolis, and the Marshfield Clinic in Wisconsin. The Harvard Pilgrim Health Care Institute in Boston shared data in the past but now just provides technical support.

The surveillance involves casting a wide net through electronic patient records, searching for specific potential adverse health effects, such as strokes or anaphylaxis (severe allergic reaction). Seven health care institutions that participate in the VSD share weekly data reports on the selected diagnoses and data analysts search for any possible connection with a COVID-19 vaccination.
Kaiser Permanente’s Vaccine Study Center has been evaluating vaccines for more than 30 years and carrying out rapid response studies through the Datalink for more than a decade, explains center Director Nicola Klein, MD, PhD. So the center has extensive experience in examining data sets, identifying specific incidents, and carrying out chart reviews to determine whether a likely adverse vaccine event took place, she said.
“The Vaccine Safety Datalink is the premier system in the United States, and arguably in the world, for active, real-time safety surveillance of vaccines,” Klein said. “Kaiser Permanente’s Vaccine Study Center is really well suited to carry out this Rapid Cycle Analysis because we are experienced doing these analyses, and actually found a safety finding in the past.” In 2008, Klein’s group identified an increase in fever seizure in toddlers who received a combination measles, mumps, rubella and varicella (MMRV) vaccine using this same Rapid Cycle Analysis approach.
Results of the ongoing analyses will be shared within the VSD network, which includes CDC representatives, during its weekly calls. Any potential safety concerns would be discussed by CDC’s Advisory Committee on Immunization Practices (ACIP), which holds regular public meetings.
The analyses will begin soon, Klein said, now that people are beginning to receive the Pfizer/BioNTech vaccine. A list of outcomes to be tracked has been made final. If concerns about new outcomes arise over time, they can be added to VSD’s surveillance list.
The analysts look for rates of particular adverse outcomes, comparing them with the number that would be typically expected in a given population. “We’ll be looking at heart attacks, for example, and it’s on the list because no one really knows if there is a risk from the vaccine,” she said. “So we’ll monitor to see if there are excess cases, and if so, whether an increased risk is associated with a COVID-19 vaccine.”
The analysis will focus on potential serious adverse health events, as opposed to mild side effects that have been reported with the Pfizer/BioNTech vaccine. Such mild side effects include headache, body aches, chills, and pain at the injection site. These side effects do not mean a patient is infected with the virus but can be an indication that their immune system is responding to the vaccine.
While the Vaccine Study Center has decades of experience in vaccine evaluation and safety surveillance, the COVID-19 vaccines are unique both in the speed with which they came into use and the national attention focused on their safety, Klein said. “The spirit of our data analyses and surveillance is the same as usual, but we expect that there will be more visibility within the VSD network, the CDC, and the public about our findings,” she said.
Other federal vaccine safety monitoring
The weekly VSD analyses are one part of the federal government’s effort to monitor the safety of the new COVID-19 vaccines as they are rolled out into general use. The CDC and Food and Drug Administration also maintain the Vaccine Adverse Events Reporting System, a database to which physicians and others can report potential vaccine adverse reactions.
The two agencies said they will also monitor vaccine safety in other ways, such as through the Veterans Affairs and Department of Defense medical systems. The CDC also devised the new V-safe mobile app which allows vaccinated people to use their mobile phones to report any reactions to a COVID-19 vaccine.
Kaiser Permanente’s Vaccine Study Center’s COVID-19 work also includes participation in a clinical trial of the Pfizer/BioNTech vaccine, which is being given to 343 patients aged 18 to 85 and 200 patients aged 12 to 18 at 2 Kaiser Permanente medical centers. They will be followed for 2 years.
Klein was appointed by California Gov. Gavin Newsom to the Western States Scientific Safety Review Workgroup, which reviews safety and efficacy of COVID-19 vaccines authorized by the FDA on behalf of California, Oregon, Washington, and Nevada. The panel recommended distribution of the Pfizer/BioNTech and Moderna vaccines in December 2020, soon after they were authorized by the FDA.
Note: Learn more about related vaccine safety work at Kaiser Permanente Washington Health Research Institute.
# # #
About the Kaiser Permanente Division of Research
The Kaiser Permanente Division of Research conducts, publishes and disseminates epidemiologic and health services research to improve the health and medical care of Kaiser Permanente members and society at large. It seeks to understand the determinants of illness and well-being, and to improve the quality and cost-effectiveness of health care. Currently, DOR’s 600-plus staff is working on more than 450 epidemiological and health services research projects. For more information, visit divisionofresearch.kaiserpermanente.org or follow us @KPDOR.




Comments (0)