Kaiser Permanente-led analysis is first large, multi-region study of real-world experience with Shringrix
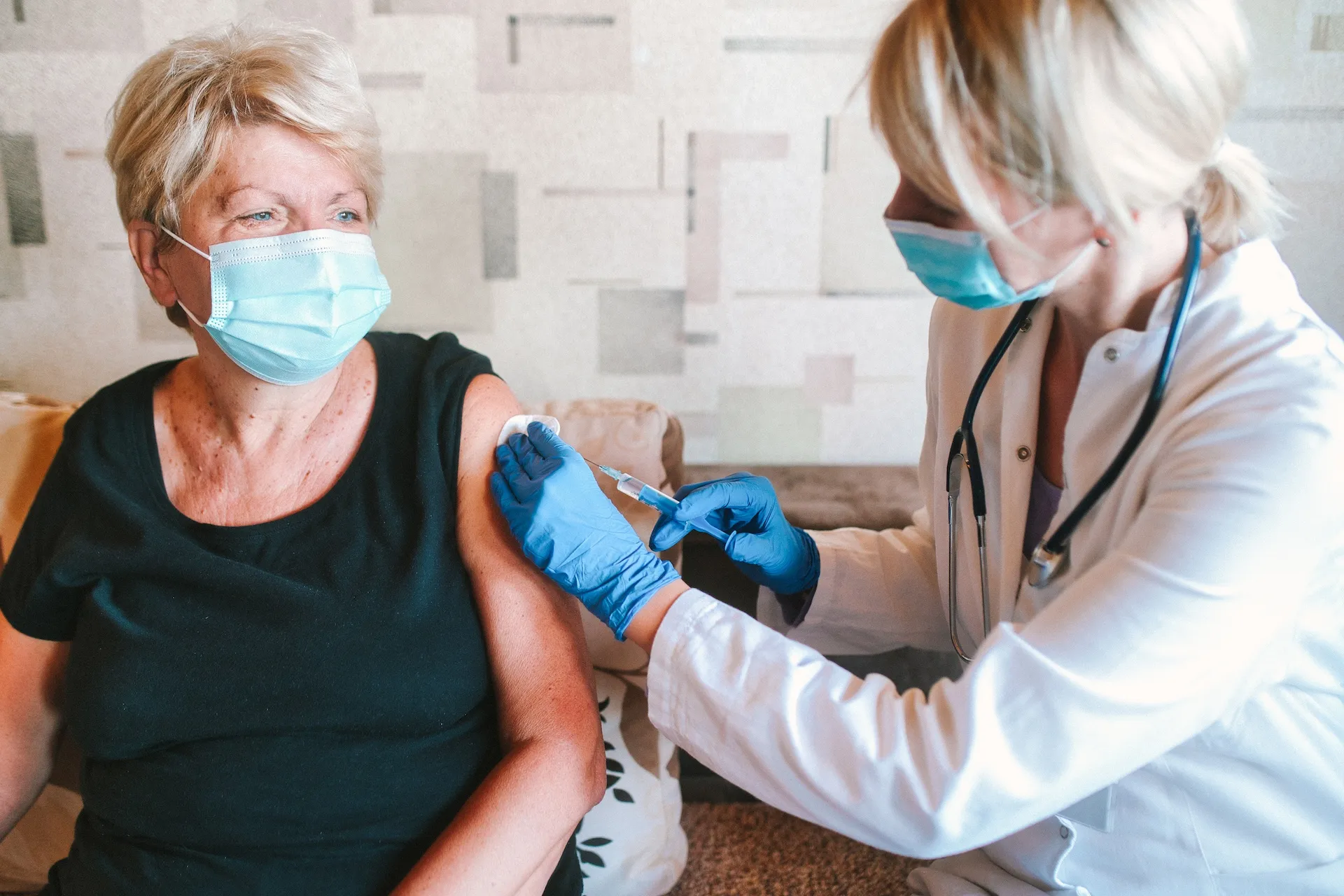
The vaccine currently used to protect people against shingles maintains strong effectiveness over 4 years post-vaccination, according to a Kaiser Permanente-led research study published in Annals of Internal Medicine.

The analysis of Shingrix vaccine effectiveness was led by the Kaiser Permanente Vaccine Study Center and included nearly 2 million patients of 4 health care systems that participate in the Vaccine Safety Datalink, a project of the Centers for Disease Control and Prevention (CDC).
Shingles is a condition also known as herpes zoster, and is a reactivation of varicella-zoster virus, which causes childhood chicken pox. Shingles can cause a painful rash and have dangerous complications, particularly if it spreads to the eyes.
Shingrix is given in 2 doses given about 6 months apart. The study found 2 doses were 76% effective against shingles, while 1 dose was 64% effective. Over the 4-year period, the 2-dose regimen waned little while the single dose waned more, dropping to 52% after the third year.
“The difference in waning between having one or two doses sends an important message about making sure you get the second dose,” said lead author Ousseny Zerbo, PhD, a research scientist with the Kaiser Permanente Division of Research.
While the CDC recommends getting the second dose within 6 months, this study found that protection after dose 2 will still be good even if that second dose is given more than 6 months after the first, said study senior author Nicola Klein, MD, PhD, director of the Vaccine Study Center. “One of the main messages is that everyone should get the 2 recommended doses, but not to panic if your second dose ended up being delayed beyond 6 months,” Klein said.
The study found Shingrix effectiveness was high, although less than that reported by the clinical trials leading to its licensing by the Food and Drug Administration in 2017. This is not surprising because clinical trials included select participants and researchers actively followed participants for shingles, Zerbo said. Previous studies on the vaccine’s effectiveness in real-world use covered a shorter time period than this study’s 4-year follow-up, he added.

The study also provided new information about the recombinant shingles vaccine’s effectiveness in immunocompromised people, finding that the vaccine had 65% effectiveness against shingles, showing that the vaccine’s protection also benefits this population. In general, immunocompromised people are at higher risk of getting shingles than those with strong immunity.
The study included data from patients vaccinated between 2018 to 2022 from 4 health systems that participate in the Vaccine Safety Datalink: Kaiser Permanente regions in Northern California, Colorado, and the Northwest, along with the Marshfield Clinic in Wisconsin.
Shingrix is a vaccine that uses recombinant technology and has been the recommended shingles vaccine in the U.S. since 2017 for people aged 50 and older. In 2021, the CDC Advisory Committee on Immunization Practices voted to recommend Shingrix to immunocompromised people ages 19 years or older.
Previously, older adults in the U.S. were given a live shingles vaccine (Zostavax), which waned significantly in the years following vaccination. Its use was dropped in 2017 when the recombinant vaccine became available. Kaiser Permanente’s Vaccine Study Center recently published an effectiveness study on Zostavax, which found significant waning over 10 years.
The study was funded by the CDC.
Additional co-authors were Joan Bartlett, MPH, Bruce Fireman, Ned Lewis, MPH, and Kristin Goddard, MPH, of the Division of Research; Kathleen Dooling, MD, and Jonathan Duffy, MD, of CDC; Jason Glanz, PHD, of the Kaiser Permanente Institute for Health Research; Allison Naleway, PhD, of the Kaiser Permanente Center for Health Research; and James G. Donahue, DVN, PhD, MPH, of the Marshfield Clinic Research Institute.
# # #
About the Kaiser Permanente Division of Research
The Kaiser Permanente Division of Research conducts, publishes and disseminates epidemiologic and health services research to improve the health and medical care of Kaiser Permanente members and society at large. It seeks to understand the determinants of illness and well-being, and to improve the quality and cost-effectiveness of health care. Currently, DOR’s 600-plus staff is working on more than 450 epidemiological and health services research projects. For more information, visit divisionofresearch.kaiserpermanente.org or follow us @KPDOR.



This Post Has 0 Comments